Q2 is in the books and it was full of electrifying news, big accomplishments and new beginnings. Of course, a major milestone was the acquisition of Shockwave Medical by Johnson & Johnson MedTech. This new journey is off to a great start and we’re excited about how this partnership will help us expand access to our life-changing technologies across the globe.
Updates from the Reducer team for our international physician partners include publishing new data that show positive news for patients suffering from Refractory Angina.* Additionally, our Size for Success training about sizing up for the best Peripheral IVL results is also gaining traction and was featured in several publications. Read the latest PulsePoint Newsletter to learn more about important milestones from Q2!
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD,
Reducer
There’s no better way to start a new year than building positive momentum. Between great developments on coronary reimbursement to positive real-world data for Reducer, 2024 is off to a shock-worthy start.
Another highlight was the continuous enrollment of patients in our EMPOWER CAD Study — the first prospective clinical study in the interventional space completely dedicated to female patients. Our team also created new resources about optimal sizing for Peripheral IVL, as well as put the final touches on our new manufacturing facility in Costa Rica. Read more about our strong start and get excited about what’s to come in the latest edition of the PulsePoint Newsletter.
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD,
Reducer
What a fantastic finish! From launching the new 120-pulse Shockwave C2+ catheter in the U.S. to establishing a new Category I CPT® Add-on Code for coronary IVL, Q4 was filled with wave-making moments and positive momentum.
One highlight was the release of sex-specific outcomes data for both coronary and peripheral IVL from the DISRUPT PAD III, DISRUPT CAD III and CAD IV studies. Additionally, the DISRUPT PAD III study provided important data supporting the practice of sizing up by 10% for peripheral IVL. Look back at a few of our most noteworthy Q4 accomplishments and get excited about what’s coming in 2024 in the latest issue of the PulsePoint Newsletter.
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD,
Reducer
From the first U.S. Shockwave C2+ case to the unveiling of the 2023 TopShock finalists, this past quarter had a lively pulse all its own. In other news, we’re very excited to share the impressive results from the 2-year DISRUPT CAD III study of IVL for Treatment of Severely Calcified Coronary Arteries.
For our international customers, there was an excellent poster session presented during ESC Congress that featured three presentations on our Coronary Sinus Reducer*. We also launched a series of real-world application videos from VAM 2023 that highlight Shockwave L6 and the benefits of using it to tackle calcium in large vessels. Dive head first into the latest PulsePoint Newsletter to learn more about the big waves made in Q3!
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD,
Reducer
Over the past three months, Shockwave continued to ride a big wave of momentum with new product launches, inclusions in new journal articles, a pulse-racing presence at EuroPCR and more. The launch of Shockwave L6 Peripheral IVL Catheter in the U.S. came with physician-led case reviews, Q&As and more valuable content that demonstrate the big impact it can make in large peripheral vessels. We also turned up the heat at EuroPCR in Paris, where we showcased live cases, hosted an engaging symposium and participated in PCR TV interviews - all while focusing heavily on the European launch of our Shockwave C2+ Coronary IVL Catheter, which includes 50% more pulses than the original Shockwave C2. Dive right in and catch the wave of excitement in the latest issue of the PulsePoint newsletter!
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD
2023 was off to a shockingly great start! We were thrilled to acquire Neovasc, a company with a first-of-its-kind technology to address refractory angina, and are looking forward to driving value for physicians and improving the lives of an underserved patient population. The excitement for our new peripheral product, Shockwave L6, was electrifying and has continued to make waves across the U.S. Shockwave IVL and Coronary IVL became listed by SCAI as a potential therapy option across all U.S. cath labs regardless of surgical backup status. As always, we were further confirming the efficacy and safety of Shockwave IVL across different calcium morphologies through new publications. We highlight these topics and more in our latest PulsePoint Newsletter!
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD
2022 was another big year for Shockwave and Q4 was no exception! On Twitter, the first Shockwave L6 cases were posted by physicians who participated in its limited release and it was great to see their excitement for our new large-vessel peripheral IVL catheter launching soon in the U.S.! At VIVA ‘22, Dr. Ehrin Armstrong presented the final 1,373 patient cohort data from the Disrupt PAD III Observational Study; this larger patient data set reinforces the predictability of Shockwave IVL and its ability to consistently modify calcium across vessel beds, challenging lesions and complex patients. Additionally, international calcium experts shared their real-world experiences with the safety and efficacy of coronary IVL across different calcium morphologies, including concentric, eccentric and nodular calcium. Check out that and more in the new PulsePoint Newsletter!
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Reimbursement,
Treating Different Ca++ Morphologies,
Female vs. Male Outcomes,
Empower CAD
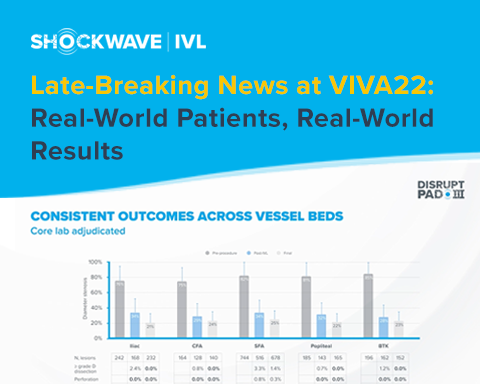
In a late-breaking clinical trial session at VIVA22, Dr. Ehrin Armstrong presented the final 1,373 patient cohort data from the Disrupt PAD III Observational Study (OS). PAD III OS represents the largest prospective ‘real-world’ evidence for the treatment of complex, heavily calcified peripheral artery disease. Building on the interim results presented at VIVA21, this larger patient data set reinforces the predictability of IVL and its ability to consistently modify calcium across vessel beds, challenging lesions, and complex patients (CLI, dialysis, and female patients). These real-world outcomes mirror the previously reported PAD III Randomized-Controlled Trial showing that IVL safely and effectively modifies challenging calcium in complex patients.
Check-out the presentation below and hear from Dr. Armstrong on the importance of the largest prospective ‘real-world’ data set in the treatment of heavily calcified PAD.
Read More
Topics:
Peripheral IVL,
Peripheral Conferences,
Peripheral Clinical Data,
Shockwave M5 & Shockwave M5+
It was smooth sailing for Shockwave this summer! Thank you to everyone who made Q3 unforgettable; but before we get cozy for the holidays, let’s reflect on the events that made the quarter one to remember. This year’s TCT was particularly momentous, as we announced the first prospective, female-only study of coronary interventions, EMPOWER CAD. We also explored tips and tricks for IVL with guide extension catheter usage with Dr. Stephan Heo and debated different approaches to modifying nodular calcium with an expert panel. Read up on the latest Shockwave IVL peer-written publications and more in the Q3 PulsePoint Newsletter.
Read More
Topics:
Coronary IVL,
Peripheral IVL,
IVL Technology,
Coronary Clinical Data,
Peripheral Conferences,
Peripheral Clinical Data,
SWAV News,
Shockwave C2,
Coronary Conferences,
Shockwave S4,
PulsePoint Newsletter,
Calcium Corner,
Shockwave M5 & Shockwave M5+,
Female vs. Male Outcomes,
Empower CAD
Join us in Las Vegas for VIVA 2022 as we crack into late breaking data supporting IVL’s consistency in calcium modification in real-world PAD. The late breaker, presented by Dr. Ehrin Armstrong, features the final 1,373 patient cohort datafrom the Disrupt PAD III Observational Study. Following the data release, an esteemed panel will discuss IVL’s consistent outcomes from both real-world AND randomized studies. Don’t roll the dice with calcium modification and join us at VIVA2022!
Read More
Topics:
Peripheral IVL,
IVL Technology,
Peripheral Conferences,
Peripheral Clinical Data