Size for Success
Peripheral IVL Sizing for Optimal Results
Though sizing 1:1 to the healthy RVD may be a customary approach seen in other devices like traditional balloon angioplasty, Peripheral IVL is an exception to the rule. It's recommended to oversize Peripheral IVL by 10% (a ratio of 1.1:1) to the healthy RVD because it achieves better and sustained wall apposition, which leads to more efficient energy transfer from the IVL device.(1, 2, 3)
Under-sizing often happens with Peripheral IVL (and other endovascular devices) due to concerns over safety and the limitations of angiography, which can result in undertreatment. However, it's important to remember that IVL operates at ultra-low pressures and is different from traditional angioplasty in that it relies on sonic pressure waves to do the calcium cracking, not mechanical force from the balloon itself. In addition, the DISRUPT PAD clinical program shows very low complication rates with IVL across peripheral vessel beds, even when the device is oversized by 10% or greater.

Undersized Peripheral IVL leads to:
• Energy loss, associated with less fracturing(1)
Optimally Sized Peripheral IVL (oversizing by 10%) leads to:
• Efficient energy transfer, associated with enhanced fracturing, improved stenosis reduction and better patency rates (1, 2, 3)
Clinical Evidence for Oversizing
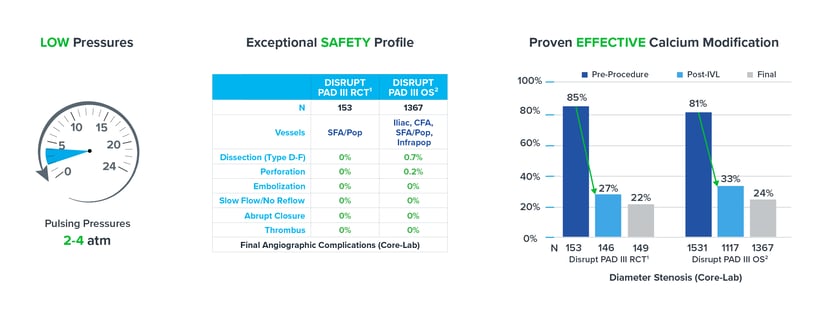
Evidence from the DISRUPT PAD clinical program (DISRUPT PAD II and DISRUPT PAD III Observational Study) shows that oversizing results in improved outcomes in terms of stenosis reduction and patency, all achieved with ultra-low treatment pressures (2-4 atm) and minimal complications, without compromising outcomes.
Improved Stenosis Reduction
• The DISRUPT PAD III Observational Study of 1,373 patients represents the largest prospective 'real-world' evidence for the treatment of heavily calcified peripheral arterial disease.
• Per a multivariable analysis, oversizing by 10% or greater was an independent predictor of improved stenosis reduction across multiple peripheral vessel beds but not a predictor of complications.(2)
Check out the DISRUPT PAD III Observational Study to learn more.
Improved Patency
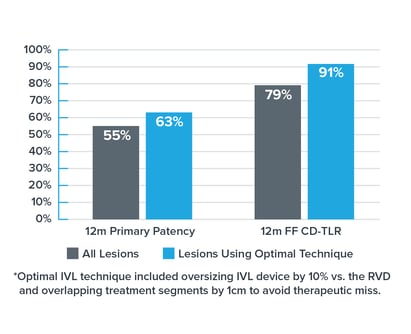
• The DISRUPT PAD II trial was a multi-center study prospectively enrolling heavily calcified, stenotic, femoropopliteal arteries with a 12-month follow-up.
• In DISRUPT PAD II, the optimal IVL technique* (including oversizing by 10% vs. the healthy reference vessel diameter) was associated with 15% improved primary patency & rate of CD-TLR in PAD II.(3)
• This technique was performed in the absence of drug, using only IVL and no drug-eluting technology.
Check out DISRUPT PAD II to learn more.
As mentioned above, Shockwave IVL works at ultra-low treatment pressures. Our data shows IVL maintains its exceptional safety profile and efficacy as a calcium modification tool even when oversized by 10%. When combined, these pillars allow you to confidently employ optimal sizing techniques in your treatment approach.
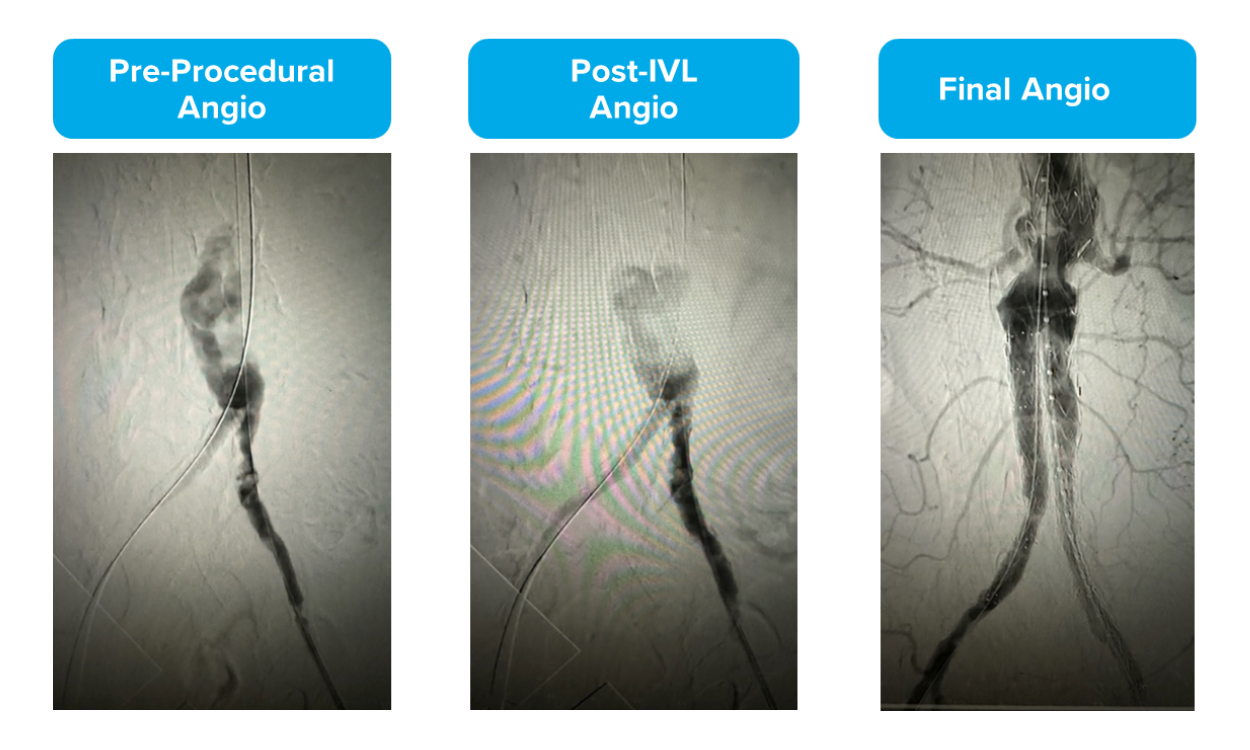
Using RVD vs. MLD for Sizing: Pre-FEVAR
By Dr. Mazin Foteh
Dr. Foteh is a paid consultant of Shockwave Medical.
As you can see, while Dr. Foteh measured the minimum lumen diameter (MLD) to be 4.5mm, the healthy reference vessel diameter (RVD) measured to 9.5mm. To optimize his treatment with IVL, Dr. Foteh used a 10.0mm Shockwave L6.
Read the article, "Adding 8-12mm diameter devices to the Shockwave Peripheral Intravascular Lithotripsy toolkit," to learn more.
Size for Success with Peripheral IVL - Interview & Case Review with Dr. Paul Foley
In this sizing discussion and case review, Dr. Paul Foley elaborates on his journey using Shockwave IVL and what led him to oversize peripheral IVL by 10% to receive optimized results.
Dr. Foley further shares his experience through reviewing three cases:
• Case 1 - Calcified Bilateral Common Iliac Artery Stenoses
• Case 2 - Calcified Left Popliteal Stenosis
• Case 3 - BTK Intervention for CLTI
Size for Success Video Series
 In this 3-part video series, Dr. Eric Secemsky (Interventional Cardiologist, Beth Israel Deaconess in Boston, MA) and Dr. Michael Siah (Vascular Surgeon, UT Southwestern Medical Center in Dallas, TX) discuss appropriate peripheral intravascular lithotripsy (IVL) sizing — the what, why and how — for optimal clinical results.
In this 3-part video series, Dr. Eric Secemsky (Interventional Cardiologist, Beth Israel Deaconess in Boston, MA) and Dr. Michael Siah (Vascular Surgeon, UT Southwestern Medical Center in Dallas, TX) discuss appropriate peripheral intravascular lithotripsy (IVL) sizing — the what, why and how — for optimal clinical results.
• Part 1: Why We Undersize Our Endovascular Tools & the Importance of IVUS
• Part 2: Peripheral IVL Sizing for Optimal Results
• Part 3: Putting Optimal Peripheral IVL Sizing Into Action - Case Review
Check out the video series on HMP Global Learning Network.
Peer-to-Peer Articles
In the latest Endovascular Today article on Sizing for Success, Drs. Sasanka Jayasuriya and Paul J. Foley discuss:
• How they size their endovascular tools
• Their experience oversizing Peripheral IVL by 10%
• Their preferred imaging modalities
• Multiple case examples highlighting their sizing approach
Now in Journal of Vascular and Interventional Radiology, Drs. Patrick Harty and Varshana Gurusamy share their experience oversizing with Peripheral IVL and share two case reviews:
• Popliteal Disease in CLTI patient
• Below-the-Knee Disease in CLTI patient
1: Kereiakes et. al. J Am Coll Cardiol Intv 2021.
2: Data on file at Shockwave Medical.
3: Brodmann et al. Catheter Cardiovascular Interv. 2018; 1-8.
4: Tepe et al, J Am Coll Cardiol Intv 2021.
5: Armstrong E, VIVA Late Breaking Clinical Trial 2022.
Important Safety Information
In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary, carotid or cerebral vasculature.
Contraindications—Do not use if unable to pass 0.014″ (M5, M5+, S4, E8) or 0.018″ (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects–Possible adverse effects consistent with standard angioplasty include–Access site complications–Allergy to contrast or blood thinner–Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)— Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. https://discover.shockwavemedical.com/ifu
Please contact your local Shockwave representative for specific country availability.



