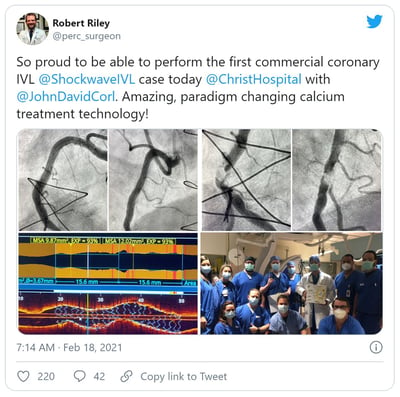
Dr. Robert Riley Q&A: 1st U.S. Coronary IVL Case
Following the recent PMA approval of coronary IVL, Dr. Robert Riley, Medical Director of the Complex Coronary Therapeutics Program, and Dr. John Corl, Medical Director of PAD/CLI Program, of the Christ Hospital in Cincinnati, performed the first commercial U.S. coronary IVL cases. Following their cases, we caught up with Dr. Riley to get his take on using the technology outside of a clinical trial and what advice he has for other physicians before they try the technology.
What was it like using the catheter outside of the clinical setting?
Dr. Riley: For some reason it was easier today. I don’t know what was different- maybe just having that experience from the DISRUPT CAD III study. It felt like it crossed easier today- seemed to cross everything without any real hitch. So I think that was the biggest difference. And then just remembering how simple it is to use.
When you think about IVL in relation to other calcium modification tools, how do you compare them?
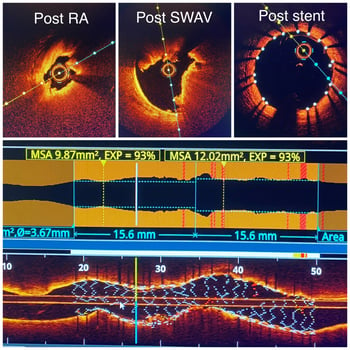
Dr. Riley: I kind of have a little bit of a hard time with this question because I know everybody wants to lump IVL with atherectomy. But you can just look at the first OCT run from the first case I did today and see how it is different. In the first case, rota created a hole with minimal fractures in the planes of the tunica intima. But there was still a thick chunk of calcium that wasn’t touched. But after IVL it showed these deep fissures all the way through the thick calcium of the tunica media throughout the entire thickness of calcium. That’s the difference. And so to me, that’s not the same. I mean these are very different technologies with different applications.
What lesions characteristics or anatomical locations do you think are going to be ideal for IVL?
Dr. Riley: My first cases haven’t changed anything that the data says. I’m going to always go back to the data because data always trumps personal experience. What I think we can hang our hat on is at least 180 degrees and at least .5mm thickness. And then length of at least 5-10mm. It’s really nice to have the calcium a little bit longer too, but frankly I think is the least predictive of the three. I think it’s really all about that arc and thickness because again, that’s where IVL offers advantages over what’s currently out there. These three characteristics seem to be relatively consistent across the algorithms that we’ve seen out of Europe.
For those who are new to the technology, what advice would you give them about starting out in their first cases?
Dr. Riley: I would say if you're going to use new technology, use all the available data points to make a decision on what you really think about it. And imaging is such a huge part of that with IVL. And then use it in real cases. Don’t pick easy stuff; use it in tough cases where you're like well this will be a tough road for orbital, rotational or whatever else. And let the technology speak for itself. I did today – and it certainly exceeded our expectations!
Important Safety Information
Rx only
Indications for Use—The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications—The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm.
Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. https://shockwavemedical.com/IFU