New U.S. Hospital Inpatient Reimbursement for Coronary IVL
New Medicare Severity Diagnosis Related Groups (MS-DRGs) With Increased Payments Replace Temporary Payment Pathway for Coronary IVL Procedures Within the Hospital Inpatient Setting
-
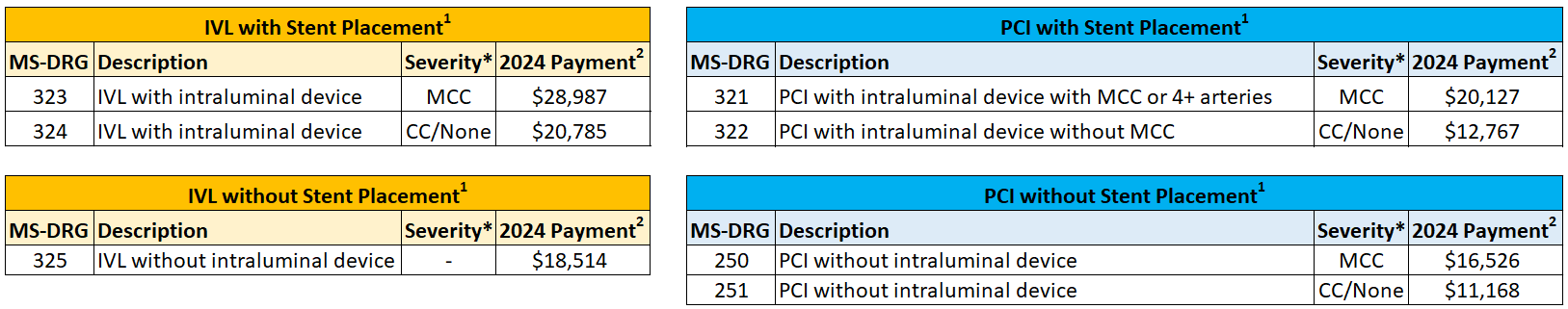
Starting October 1, 2023, three new Coronary IVL specific MS-DRGs have been created for Percutaneous Coronary Intervention (PCI) procedures involving Coronary IVL in the hospital inpatient setting. The new Coronary IVL MS-DRGs are associated with higher payments than previously available for IVL under the New Technology Add-On Payment (NTAP) program, and are higher than payments for other PCI procedures. The new Coronary IVL MS-DRGs are the first new additions for the field of PCI in over 20 years.
Additionally, the FY2024 Hospital Inpatient Final Rule1 rule consolidates what was previously four MS-DRGs involving PCI with implant of a stent into two MS-DRGs, removing the distinction between stent type – Drug Eluting Stent (DES) or Bare Metal Stent (BMS). Please see the below table for more information:
-
-
*MCC: Major Complications and Comorbidities; CC: Complications and Comorbidities
To date, hospitals have been reimbursed for Coronary IVL via a New Technology Add-On Payment (NTAP), which provided an incremental payment of up to $3,666 in addition to the relevant MS-DRG payment for a PCI procedure. The NTAP for Coronary IVL will expire at the end of FY2023 on September 30, 2023.
Please refer to the Hospital Inpatient coding guide linked below for additional context and background regarding these updates.
-
-
1. CMS-1785-F; Medicare Inpatient Prospective Payment System Fiscal Year 2024 Final Rule, Table 5
-
- 2. National Average MS-DRG rates shown are based on Medicare Inpatient Prospective Payment System FY2024 Final Rule, Table 5. National average payment rates assume full update amount for hospitals which have submitted quality data and hospitals have a wage index greater than 1. Site specific payment rates will vary based on regional area wage differences, teaching hospital status, indirect medical education costs, quality data, additional payments to hospitals that treat a large percentage of low income patients (“disproportionate share payments”), etc.
-
-
-
-
To see outstanding Shockwave IVL cases, follow @ShockwaveMedical on Instagram!
To keep up on the real-time Shockwave IVL activities, follow @ShockwaveIVL on Twitter
-
Coronary Important Safety Information:
In the United States: Rx only.
Indications for Use—The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications—The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm.
Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. https://shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave C2 instructions for use containing important safety information.





