Insights from the IVL Meta-Analysis: Q&A with Dr. Sahil Parikh
Intravascular Lithotripsy (IVL) has been used and studied across all peripheral vessels, with multiple published studies in CFA, SFA, Popliteal and Infrapopliteal arteries.
We spoke with Dr. Sahil Parikh, corresponding author on the meta-analysis, Interventional Cardiologist and Director of Endovascular Services at Columbia University, about the outcomes of the patient-level meta-analysis and the importance of the results.
Read the Meta-Analysis Publication Here: https://onlinelibrary.wiley.com/doi/10.1002/ccd.28729
Question: What is unique about this pooled analysis of IVL outcomes?
Dr. Parikh: This is the first patient-level, 100% core-lab adjudicated, prospectively-collected pooled analysis in the peripheral vascular space. The pooled analysis includes five studies, comprising over 336 patients (354 lesions) treated with IVL and gives us insights across a broad range of variables and subgroups on the safety and effectiveness with this novel technology.
Question: What were the results of the pooled analysis?
Dr. Parikh: First off, the safety of this device across all vessel beds and calcium severity was excellent. Out of 358 lesions, we observed 3 flow-limiting dissections (0.9%), 1 perforation (0.3%) and no other peri-procedural complications. Overall, the safety profile is at least comparable, but likely favorable, to other endovascular techniques for the treatment of calcified PAD.
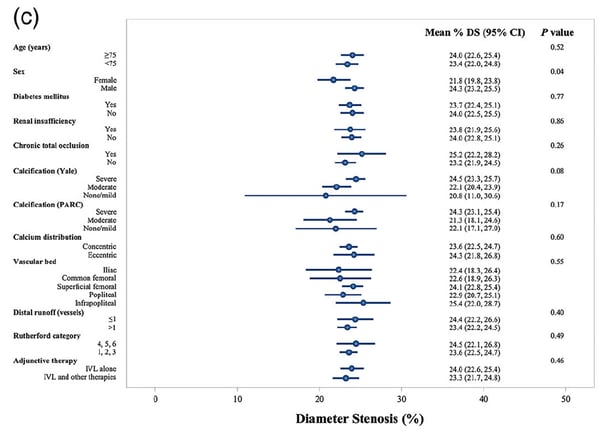
We also found acute clinical effectiveness to be highly favorable. Overall, diameter stenosis was reduced from 78.8% to 23.7%. Particularly interesting is the results of the subgroup analyses. We examined final diameter stenosis across all tested groups (presence of CTO or not), CLI vs. claudicants, vessel bed (CFA, SFA, Popliteal and Infrapopliteal) and eccentric vs. concentric calcium. There were no significant differences across any of those groups, reinforcing the consistency of the results.
Question: Were any of the results surprising or unexpected to you?
Dr. Parikh: All five of the studies included in this pooled analysis enrolled moderate-severely calcified lesions by PARC (a stringent calcium definition). These are the difficult patients that have often been excluded from contemporary studies due to their disease severity. In this context, both the safety and effectiveness of these data is quite remarkable. In particular, I was surprised to see the consistency of outcomes even in large, eccentrically calcified lesions such as those in the Iliac and CFA. In fact, there were consistently good outcomes across eccentric and concentric lesions across all vessel beds.
Question: What do you consider the key take home messages from this study?
Dr. Parikh: We found that IVL is consistently safe and effective across all studied peripheral vessel beds. We investigated various subgroups, including eccentric vs. concentric calcification, calcium severity and CLI vs. claudicants, and we found no differences in either safety or effectiveness across any of these groups.
To learn more about Shockwave IVL, register on our website for important news and follow us on Twitter @ShockwaveIVL to see the latest cases, publications, and updates on the technology.
Important Safety Information
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Indication for Use – The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications – Do not use if unable to pass 0.014 guidewire across the lesion • Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings – Only to be used by physicians who are familiar with interventional vascular procedures • Physicians must be trained prior to use of the device • Use the Generator in accordance with recommended settings as stated in the Operator’s Manual
Precautions – Use only the recommended balloon inflation medium • Appropriate anticoagulant therapy should be administered by the physician • Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology
Adverse Effects – Possible adverse effects consistent with standard angioplasty include: • Access site complications • Allergy to contrast or blood thinners • Arterial bypass surgery • Bleeding complications • Death • Fracture of guidewire or device • Hypertension/Hypotension • Infection/sepsis • Placement of a stent • Renal failure • Shock/pulmonary edema • Target vessel stenosis or occlusion • Vascular complications. Risks unique to the device and its use: • Allergy to catheter material(s) • Device malfunction or failure • Excess heat at target site
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions, and adverse events. www.shockwavemedical.com


