EuroPCR 2022: We’re back to crack…in person!
After two long years, EuroPCR is finally live again! We can’t wait to meet you in Paris and discuss the latest on IVL.
If you hold the belief that Shockwave IVL only works well in certain types of calcium, we have news for you! Join our symposium for discussion on the use of IVL in multiple calcium morphologies and the benefit of IVL in the female population. Also, attend the Tools & Techniques case-based session to see how IVL is being used in daily practice to treat calcified lesions.
Let’s crack some calcium at EuroPCR 2022!
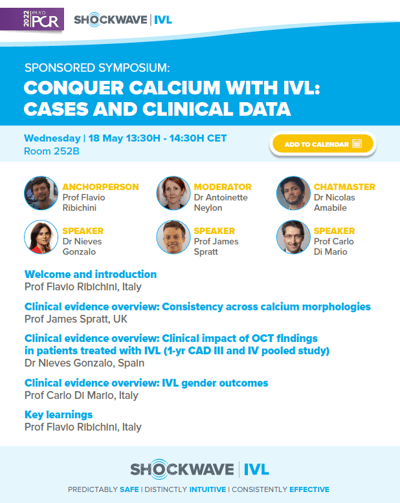
CONQUER CALCIUM WITH IVL: CASES AND CLINICAL DATA
Wednesday 18 May – 13:30-14:30 CET | ROOM 252B
In this year's sponsored symposium, Dr. Ribichini, Dr. Neylon and Dr. Amabile will guide an interesting conversation about Shockwave IVL usage in different calcium morphologies and its great outcomes on female patients.
With the experience of Drs. Di Mario, Spratt and Gonzalo, we will go through the latest clinical evidence and relevant case examples.
Finally, it’ll be valuable to hear them share their perspective on how Shockwave IVL has been impacting their practice in calcium treatment.
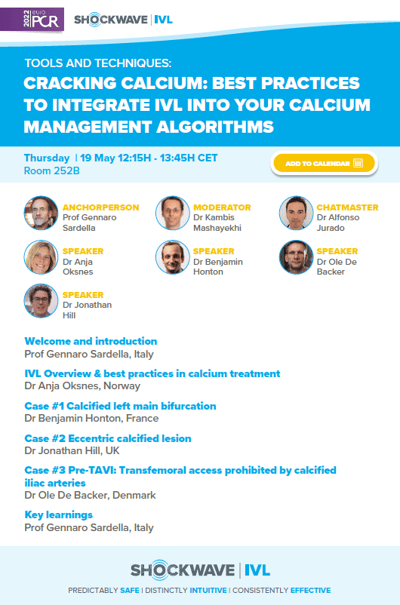
CRACKING CALCIUM: BEST PRACTICES TO INTEGRATE IVL INTO YOUR CALCIUM MANAGEMENT ALGORITHMS
Thursday 19 May – 12:15-13:45 CET | Room 252B
Shockwave IVL Tools and Techniques session will be all about best practices and how to optimize application of intravascular lithotripsy in coronary vasculature.
With the experienced guidance of Prof. Sardella, Dr. Mashayekhi and Dr. Jurado-Román, we will go through three virtual live cases that will show how IVL is incorporated in calcium management algorithms to improve outcomes. Two coronary cases performed by Dr. Honton and Dr. Hill, and a pre-TAVI IVL case with the new Shockwave M5+ catheter performed by Dr. De Backer.
To keep up on the real-time Shockwave IVL activities at EuroPCR 2022, follow @ShockwaveIVL on Twitter!
Important Safety Information
Coronary Important Safety Information:
In the United States: Rx only.
Indications for Use—The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications—The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm.
Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. https://shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave C2 instructions for use containing important safety information.
Peripheral Important Safety Information:
In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications—Do not use if unable to pass 0.014 guidewire across the lesion—Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as
stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects–Possible adverse effects consistent with standard angioplasty include – Access site complications – Allergy to contrast or blood thinner – Arterial bypass surgery — Bleeding complications — Death — Fracture of guidewire or device — Hypertension/Hypotension — Infection/sepsis — Placement of a stent — renal failure — Shock/pulmonary edema — target vessel stenosis or occlusion — Vascular complications. Risks unique to the device and its use — Allergy to catheter material(s) — Device malfunction or failure — Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave S4, Shockwave M5, and Shockwave M5+ instructions for use containing important safety information.