Q&A with Dr. Chandan Devireddy on the use of IVL to facilitate transfemoral TAVR in calcified iliacs
At the EPIC-SEC conference in May 2019, Dr. Chandan Devireddy performed a live case using Shockwave IVL in advance of a TAVR procedure. He tweeted about the procedure and has offered to share some additional context about the specific case, as well as the potential impact of IVL to facilitate transfemoral TAVR in the presence of calcified iliac access in general.
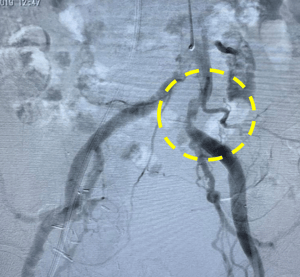
The patient was an 84 year old man with symptomatic severe AS, NYHA class III. He received a renal transplant vascularized by an artery anastomosed to right external iliac artery. A 29mm Medtronic Evolut R TAVR device was planned through the left femoral artery. Shockwave IVL was used in the left iliac artery in advance of TAVR delivery. The device was advanced and the valve was implanted successfully. There was no dissection or other adverse event and iliac artery with patent with no more than 30-40% residual stenosis following IVL.
Left Image: Preprocedural angiogram.
Above: IVL Delivery (left), Sheath Advancement (right).
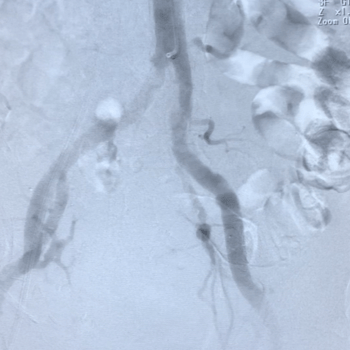
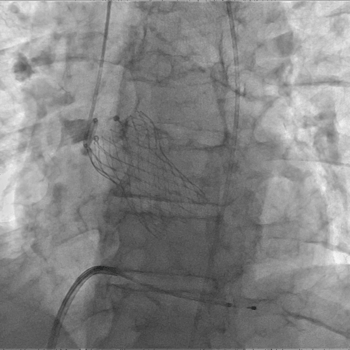
-
Above: Post-IVL (left), Successful 29mm Evolut R Implantation (right)
Q & A with Dr. Chandan Devireddy
1. What are the challenges associated with calcified Iliac access? How common is it?
TAVR devices are most commonly and preferably delivered using the transfemoral approach. However, significant calcium in the iliac arteries can prevent safe delivery of these large devices, which typically require a vessel lumen of at least 5.5mm. Adjunctive maneuvers may sometimes allow for safe transfemoral TAVR delivery in the presence of calcium, but often these patients end up requiring some form of non-femoral access (i.e., transcaval, transcarotid, axillary/subclavian). These approaches are required in approximately 8-10% of patients.
- 2. What alternative approaches would you have considered for this patient in order to deliver a transcatheter valve for this patient?
We have attained proficiency in the transcaval approach to alternative TAVR access. This approach has proven to be a safe alternative to transfemoral access for those patients who have prohibitively calcified iliac access, but have an appropriate aortic “window” to pass the TAVR device through. While this technique has allowed us to deliver transcatheter valves in these challenging patients, it carries with it a significant learning curve, significant device cost and a longer hospital length of stay than the standard transfemoral approach. There are questions regarding increased bleeding risk which we are continuing to study.
- 3. How well did IVL work for this case and what did it help you achieve?
IVL was deployed in a highly calcified and angulated segment that would not have allowed passage of a TAVR device safely. The device was easy to use – preparation is similar to a standard PTA balloon, the generator is battery powered and requires no warm up time and the device is deployed like a standard balloon over any 0.014” wire. After minutes of energy delivery, the significantly calcified segment visibly opened up. The TAVR device was safely delivered through the calcified region, the valve was successfully deployed and the sheath was withdrawn without any complications. In this case, IVL allowed us to safely deliver the TAVR device using the preferred transfemoral approach with minimal additional cost and procedure time.
- 4. What makes IVL a good solution for some patients with prohibitively calcified iliac access?
IVL is easy to learn, easy to use and has proven to be very safe. The transcaval approach will still be necessary for some patients with prohibitive access (especially in those with small or highly tortuous anatomy), but IVL may prove to be a preferable strategy, preserving the well-documented benefits of transfemoral TAVR delivery in a challenging patient population.
Important Safety Information
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Indication for Use – The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications – Do not use if unable to pass 0.014 guidewire across the lesion • Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings – Only to be used by physicians who are familiar with interventional vascular procedures • Physicians must be trained prior to use of the device • Use the Generator in accordance with recommended settings as stated in the Operator’s Manual
Precautions – Use only the recommended balloon inflation medium • Appropriate anticoagulant therapy should be administered by the physician • Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology
Adverse Effects – Possible adverse effects consistent with standard angioplasty include: • Access site complications • Allergy to contrast or blood thinners • Arterial bypass surgery • Bleeding complications • Death • Fracture of guidewire or device • Hypertension/Hypotension • Infection/sepsis • Placement of a stent • Renal failure • Shock/pulmonary edema • Target vessel stenosis or occlusion • Vascular complications. Risks unique to the device and its use: • Allergy to catheter material(s) • Device malfunction or failure • Excess heat at target site
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions, and adverse events.www.shockwavemedical.com


