2024: Improved Reimbursement to Expand Treatment Options in Germany
For patients with calcified PAD with Peripheral Shockwave Intravascular Lithotripsy (IVL)
Since the introduction of Shockwave Peripheral Intravascular Lithotripsy (IVL) to the market in Germany there has been a significant underfunding (OPS code 8-83c.bb, 8-83c.bc, 8-83c.b9) when used in percutaneous peripheral vascular procedures. Following the annual review of the DRG catalogue, INEK has recognized this underfunding and changed the DRG which Shockwave IVL procedures track to from F59E to F59D. This change results in a € 1.258,35 increase in reimbursement increasing from € 3.676,65* (F59E in 2023) to € 4,935.00† (F59D in 2024), for procedures where IVL is used.
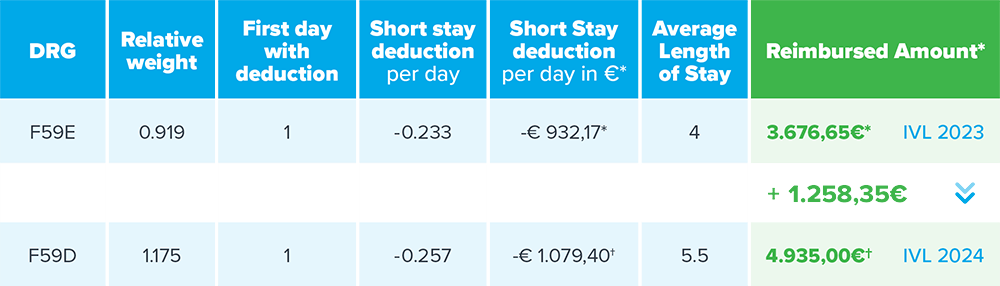
*calculated with federal base rate 2023 of € 4.000,71 (nursing payment not included). The federal base rate for 2024 has not yet been published.
†calculated with BVMed estimated 2024 base rate of € 4,200 (care supplement not included). The federal base rate for 2024 has not yet been published.
With these positive changes implemented, any peripheral vascular intervention now performed using Shockwave Peripheral IVL; whether it be in the Iliac, Femoropopliteal or Tibial arteries, will in general track to the DRG F59D. The use of Shockwave IVL alongside other OPS codes - or combinations of OPS codes - triggering higher reimbursement will not limit reimbursement to F59D. The improvements to Shockwave IVL reimbursement will allow physicians to utilise a technology reinforced by excellent safety and efficacy data and provide their patients with an improved standard of care in calcified PAD treatment.
The reimbursement amounts in this document are calculated based on the BVMed estimated base rate for 2024 of EUR 4,200. Individual hospital reimbursement is calculated according to the applicable state base rate, which may be lower than the federal base rate. The below statements apply to treatment in hospital main department (Hauptabteilung). Transfers to another hospital have not been considered. Shockwave Medical cannot guarantee success in obtaining third-party insurance payments. Third-party payment for medical products and services is affected by numerous factors. It is always the provider's responsibility to determine and submit appropriate codes, charges, and modifiers for services that are rendered. Providers should contact their third party payers for specific information on their coding, coverage and payment policies.
Important Safety Information
Peripheral IVL
Shockwave M5+, Shockwave M5, Shockwave S4 and Shockwave L6 Safety Information
Indications for Use —The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications — Do not use if unable to pass 0.014" (M5, M5+, S4) or 0.018" (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings — Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator's Manual.
Precautions — Use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects — Possible adverse effects consistent with standard angioplasty include—Access site complications—Allergy to contrast or blood thinner—Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—Renal failure—Shock/pulmonary edema—Target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)—Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability.


