2022 Medicare Reimbursement Updates
Great news! The Centers for Medicare and Medicaid Services (CMS) has increased payment for peripheral IVL procedures. The increases apply to procedures performed in anatomical locations above the knee in a hospital outpatient setting. The changes go into effect on January 1, 2022.
In the 2022 Medicare Final Rule for Hospital Outpatient Payment2, CMS announced new Ambulatory Payment Classifications (APC) assignments for peripheral IVL procedures. The table below shows the HCPCS3 codes for peripheral IVL procedures above the knee and the corresponding changes in APC assignments and payments.
 Table: New APC Assignments for above the knee Peripheral IVL HCPCS codes
Table: New APC Assignments for above the knee Peripheral IVL HCPCS codes
The payment increases apply to IVL procedures performed in the iliac, common femoral, SFA or popliteal arteries both when IVL is performed as a stand-alone therapy or adjunctively with PTA, DCBs, stents or atherectomy.
The table below summarizes 2022 Medicare payments in the hospital outpatient setting for common PAD interventional treatment strategies for iliac and fempop arterial territories. The table stratifies 2022 Medicare payments for hospitals by common vessel preparation strategies (balloon-based, atherectomy, IVL) per the most common definitive treatment strategies (DCB, stents) utilized in the iliac and fempop arterial territories.
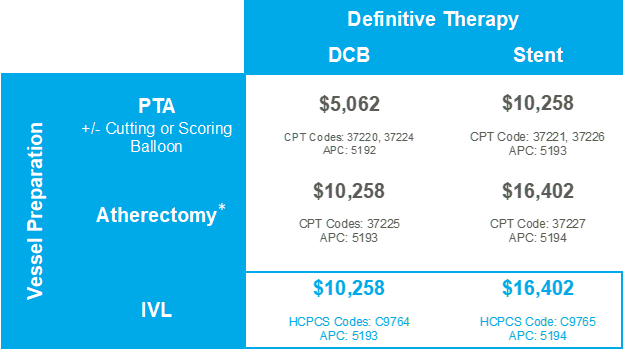
*Most atherectomy not indicated for iliacs, coding available but coverage is inconsistent and hospital-specific.
IVL is indicated for and reimbursed in the iliacs.
With the increase in APC assignments, Medicare payments for peripheral IVL procedures are now in line with other treatment strategies utilized in complex lesions and reflective of the additional resources required to treat heavily calcified lesions. And now that hospital payments have been aligned with costs, physicians and hospitals can make treatment decisions based on which therapies best meet the needs of the clinical situation.
It is important to note that hospitals need to utilize the peripheral IVL HCPCS codes noted above in order to receive the Medicare payments detailed in this document. Please follow this link to access Shockwave’s 2022 Medicare Quick Reference sheet and 2022 Coding and Reimbursement Guide. For additional information, please contact reimbursement@shockwavemedical.com or call the Shockwave Reimbursement Hotline at (877) 273-4628. The Shockwave Field Reimbursement Team can provide resources to help hospital coding and revenue teams understand the reimbursement available for Shockwave peripheral and coronary therapies.
To learn more about Shockwave IVL, follow @ShockwaveIVL on Twitter!
Footnotes
1 Current Procedural Terminology. Copyright 2021 American Medical Association. All rights reserved. Applicable FARS/HHSARS apply.
2 CY 2022 OPPS Final Rule. CMS-1753-FC (86 Fed. Reg. 63458 (Nov. 16, 2021) OPPS Addenda A and B, Ambulatory Surgery Center (ASC) Addenda AA, and BB, available at https://www.cms.gov/medicaremedicare-fee-service-paymenthospitaloutpatientpps/cms-1753-fc
3 HCPCS = Healthcare Common Procedures Coding System
4 Medicare 2021 OPPS Final Rule is available for download here: https://www.cms.gov/files/document/12220-opps-final-rule-cms-1736-fc.pdf
5 Atherectomy not indicated for use in iliac arteries. No HCPCS or CPT codes available
Important Safety Information
In the United States: Rx only
Indications for Use – The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications – Do not use if unable to pass 0.014 guidewire across the lesion • Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings – Only to be used by physicians who are familiar with interventional vascular procedures • Physicians must be trained prior to use of the device • Use the Generator in accordance with recommended settings as stated in the Operator’s Manual
Precautions – Use only the recommended balloon inflation medium • Appropriate anticoagulant therapy should be administered by the physician • Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology
Adverse Effects – Possible adverse effects consistent with standard angioplasty include: • Access site complications • Allergy to contrast or blood thinners • Arterial bypass surgery • Bleeding complications • Death • Fracture of guidewire or device • Hypertension/Hypotension • Infection/sepsis • Placement of a stent • Renal failure • Shock/pulmonary edema • Target vessel stenosis or occlusion • Vascular complications. Risks unique to the device and its use: • Allergy to catheter material(s) • Device malfunction or failure • Excess heat at target site
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions, and adverse events. www.shockwavemedical.com
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave S4, Shockwave M5 and Shockwave M5+ instructions for use containing important safety information.



